III.3.E. The effect of the growth hormone on the blood glucose level
The GH is released from the adenohypophysis. GH bound to GH-binding protein (GHBP) circulates in the blood.
Its release follows a circadian rhythm; the highest level is released in the first hour of slow-wave sleep. Its level changes during life; over 20 years, the concentration falls continuously.
The hypothalamic GH-releasing hormone (GHRH) and ghrelin induce, while Somatostatin inhibits GH production (Fig. 8). Ghrelin is released from the hypothalamus or from the intestines during a meal.
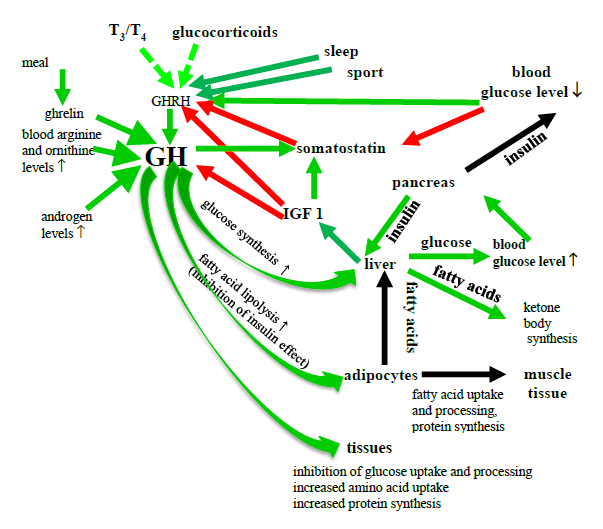
Figure 8. The effects of GH on the blood Glucose level
(T3 = triiodothyronine, T4 = thyroxine, GH = growth hormone, IGF-1 = insulin-like growth factor 1, GHRH = GH-releasing hormone red arrow = inhibition; green arrow = stimulation, green dashed arrow = permissive role)
The release of the GH is influenced by various factors:
It is stimulated by a decrease in the blood Glu level, an increased blood FFA or amino acid level, anorexia nervosa, physical activity and sleep.
It is inhibited by obesity and sex hormones.
GH is an insulin antagonist. It inhibits glycogen synthesis and Glu oxidation, and stimulates the liver Glu production, thereby increasing the blood Glu level.
GH enhances fat breakdown (lipolysis), and hence increases the blood FFA level. Energy depletion and starvation enhance these effects of GH. These are probably indirect effects, through the stimulation of catecholamine effects.
GH increases protein synthesis, enhances renal water and Na+ reabsorption, and therefore increases the BP, and stimulates the SNS, so that it may cause headaches.
Various diseases develop as a result of the over- or underproduction of GH.
The underproduction of GH (GHD, growth hormone deficiency) causes metabolic syndrome-like symptoms. The muscle mass is reduced, and central obesity may develop (because of the lack of GH-stimulated abdominal lipolysis). GHD is a risk factor for CVDs: the left ventricular muscle mass, the ejection fraction, the duration of diastolic filling and the contraction of the myocytes are decreased; the diastolic BP and blood clots are increased.
The above-mentioned decreases the level of physical fitness of obese patients, and this leads to a sedentary lifestyle, and further enhances symptoms and development of the MetS.
The level of released catecholamine is decreased. Insulin resistance and dyslipidemia develop. The risk of oxidative stress and inflammation increases (the levels of TNF-α and IL-6 are increased), and the function of the endothelium decreases (the level of LPL increases, which results in the development of atherosclerosis).
The overproduction of GH leads to the developments of acromegaly, DM and high BP. Acromegaly causes an increase in the left ventricular mass within a few years, an increase in the cardiac output, disturbances in the cardiac conduction and an irregular heartbeat. (The coronaries of these patients do not increase, and this leads to heart attack.) The increased GH level enhances the thickening of the blood vessel wall, and further elevates the high BP.
IGF-1 (also known as somatomedin C) is responsible in part for the effects of GH. IGF-1 is released from the liver, muscle, intestines and kidneys; binding of the hormone to the receptor initiates a series of events. IGF-1 inhibits the releases of GHRH and GH by a negative feedback mechanism.
IGF-1 knockout mice grow normally, but are insulin-resistant. IGF-1 stimulates peripheral Glu uptake and glycogen synthesis, but it does not influence the Glu metabolism in the liver. It exerts its effects more slowly than does insulin. It has protein anabolic effects, increases lipolysis in the adipose tissue, and decreases peripheral Glu consumption, thereby increasing blood Glu level.
There is an interesting overlap between the effects of IGF-1 and insulin on the protein metabolism. IGF-1 in a physiological amount increases protein synthesis, and insulin inhibits protein breakdown. Above the physiological concentration, IGF-1 inhibits protein breakdown, but insulin stimulates protein synthesis.
The circulating IGF-1 half-life is very short (2-5 min), but when IGF-1 binds to its specific binding protein (insulin-like growth factor-binding globulin, IGFBP), the half-life may be 16 h.
Obesity decreases the amount of IGFBP, but increases the amount of GHBP. The level of IGFBP is decreased in MetS patients.
Insulin stimulates IGF-1 synthesis in the liver. IGF-1 release is impaired in DM patients, and GH regulation is therefore also impaired. This is the cause of “early morning symptoms”: DM patients are less sensitive to insulin, and the plasma Glu level is lower in the morning. This may be caused by lower IGF-1-induced GH overproduction in the night.
IGF-1 has a vasodilator effect. A decreased level of IGF-1 is a risk factor for heart attack or for blood clots.