The NOS-catalyzed reaction
Biosynthesis of NO involves a two-step oxidation of L-arginine to L-citrulline, with concomitant production of NO (Fig.11). The reaction consumes 1.5 mol of NADPH and 2 mol of oxygen per mol of L-citrulline formed. The proposed mechanisms are discussed in length by Griffith and Stuehr and others, and involve an initial hydroxylation of L-arginine, leading to the formation of NG -hydroxy-L-arginine, which can also act as a substrate for NOS. This is followed by oxidation of the intermediate (N ω-hydroxy-l-Arg (NOHA)), using a single electron from NADPH, to form L- citrulline and NO. The enzyme is also capable of catalysing the production of additional products, notably superoxide anion (O2-), depending on the conditions.
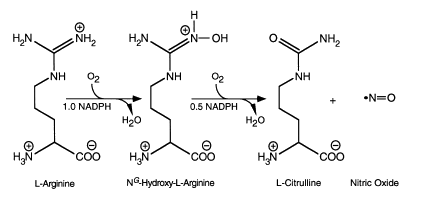
Figure 11: The NOS-catalysed reaction