Heme-oxygenase enzyme (HO)
HO enzyme serves as the rate-limiting enzyme in the degradation of heme. HO catalyzes the first and rate-limiting step in the breakdown of heme to yield equimolar quantities of biliverdin IXa, CO, and iron (Fig. 4A and 4B).
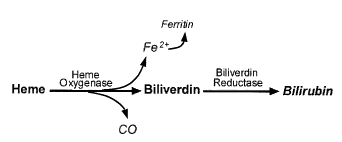
Figure 4A: Catalytic reaction of heme oxygenase. CO indicates carbon monoxide
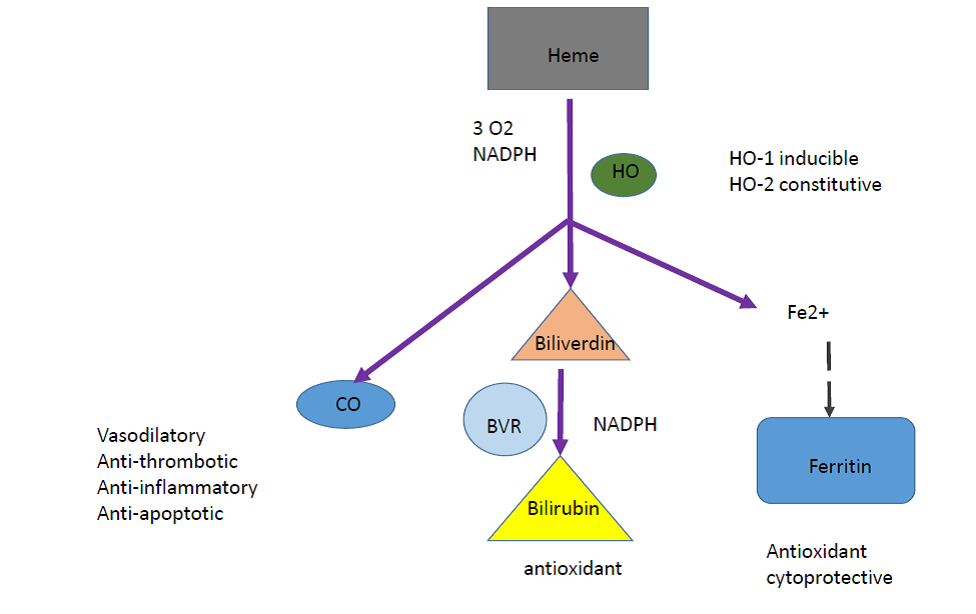
Figure 4B: Catalytic reaction of heme oxygenase
The heme oxygenase (HO) enzyme system generates the majority of endogenous CO. Biliverdin is subsequently converted to bilirubin through the action of biliverdin reductase, and free iron is promptly sequestered into ferritin. There are 3 isoforms of HO: HO-1 is highly inducible, whereas HO-2 and HO-3 are consititutively expressed. The major substrate of HO-1 is heme, however a variety of nonheme agents including: heavy metals, cytokines, hormones, endotoxin, and heat shock are also strong inducers of HO-1 expression.
Also, HO-1 is highly induced by a variety of oxidative stress molecules including: hydrogen peroxide, glutathione depletors, UV irradiation, endotoxin, hypoxia, and hyperoxia. One interpretation of this finding is that HO-1 can serve as a key biological molecule in the adaptation and/or defense against these oxidative and cellular stresses.
In addition, recent studies of HO-1-/- null mice has strengthened the evolving paradigm that HO-1 is indeed an important molecule in the host’s defense against cellular stress in that HO-1-/- null mice exhibited increased susceptibility to endotoxin.
Furthermore the overexpression of HO-1 can confer marked cytoprotection against ischemia/reperfusion-induced myocardial tissue injury. Mechanism(s) by which selective overexpression of HO-1 in cardiac tissue and other tissue injuries conferred protection against ischemia/ reperfusion injury remains poorly understood. All three catalytic by-products of HO-catalized heme, namely bilirubin, ferritin from the sequestration of free iron, and CO, play critical roles in mediating cytoprotection against ischemia/reperfusion tissue injury.
According to previous studies, HO-1-/- null mice exhibited increased right ventricular infarcts and increased lipid peroxidation and oxidative damage in right ventricular cardiomyocytes when compared with wild-type HO-1+/+ mice. The cytoprotective role of HO-1 in vascular injury was further supported by a recent report by Christou et al, who demonstrated an important role of HO-1 in the prevention of hypoxia-induced pulmonary hypertension. Another in vivo vascular injury model research done by Soares and colleagues demonstrated that CO not only can confer protection as effectively as HO-1, but can also confer cytoprotection in the absence of HO-1.
It is plausible that the three catalytic by-products of HO-1 act in concert and synergize with each other to optimize cytoprotection, depending on cell type and models of injury. The beneficial effect of these mediators has been shown in cultured endothelial cells, with CO and ferritin each imparting additive cytoprotection against tumor necrosis factor-α (TNF-α)–induced apoptosis. It has been shown that antioxidant bilirubin protect isolated perfused rat hearts. Though it is still not known whether ferritin can directly mediate cytoprotection against ischemia/reperfusion tissue injury, ferritin is a known cytoprotective molecule in the vascular endothelium against oxidative stress. Some observations suggested that CO may impart potent anti-inflammatory and antiapoptotic effects via the mitogen kinase pathway in macrophages and endothelial cells, respectively. These cytoprotective effects involve neither the guanylyl cyclase– cGMP nor the NO pathway.
The complexity of the signaling pathways by which CO imparts cytoprotection is further highlighted by recent reports demonstrating the critical role of cGMP in mediating antifibrinolytic and antiproliferative effect of CO in ischemia/reperfusion and vascular injury models.
What is protective and non-protective about HO?
Carbon monoxide (one CO molecule is released from each heme) act as:
- Vasodilator
- Bronchodilator
- Anti-fibrinolytic
- Anti-inflammatory
Negative effects of CO:
- Toxic gas
- Increases apoptosis
Bilirubin acts as:
- Antioxidant with some significant toxicities
Sequestration of heme:
- Removal of a pro-oxidant
- Co-induced ferritin sequesters heme iron released from the reaction