NADPH oxidase isoforms
Ever since the first NADPH oxidase was documented, several isoforms have been described and characterised into two major groups, the Nox family (contains five members, Nox 1-5) and dual oxidases (Duox1 and Duox 2) (Fig.7). The functions of these isoforms are the same, but their structure is slightly different. (Fig. 6) The first identified NADPH oxidase was the Nox2 isoform, whose subunits were previously described. In the following isoforms the subunits slightly differ from the prototypic NADPH oxidase, these are homologous to the Nox2 subunits. In Nox1 and Nox3, the binding organizer and activator proteins are NOXO1 (NOX organizer 1), which is a p47phox homolog, and NOXA1 (NOX activator 1), a p67phox homolog subunit. (NOX, NOX who is there? The contribution of NADPH oxidase one to beta cell dysfunction). Takeya et al. have found that p40phox is not necessary for the function of Nox1, and other investigations pointed out that it is not important for the Nox3 isoform either. Furthermore, there are controversal evidence about Nox3 whether Rac dependent or independent, and it is still not known how the activation occurs, whether constitutively or activation dependently. The Nox4 isoform cytosolic subunits are not required for its activation, this enzyme might be a constitutively expressed isoform. Several studies described that Nox4 generates a high amount of H2O2 rather than O2-, but this happens because of its localization. The Nox4 is localizated in intracellular organelles, and the generated O2- is further converted to H2O2 by superoxide dismutase (SOD). The Nox5 isoform developed by Cheng et al. and Banfi et al., differs from the previously described enzymes, because it does not require a p22phox transmembrane domain nor cytosolic subunits, however, it contains an EF hand motif on its cytosolic site. The p22phox independence is proven by siRNA supression of this subunit, which decreases the activity of Nox1-Nox4, but does not influence the Nox5 isoform’s activity. The Nox5 activation is based on a protein-protein interaction between the catalytic C terminus and the EF hand Ca2+ binding domain. The Nox5 regulation is realized by the increase of the cytosolic Ca2+ concentration. Recently discovered isoform are the dual oxidases, DUOX1 and DUOX2, which were identified from tyroid gland. In additon to Nox1-4 homology subunit and an EF hand structure, DUOX proteins contain a seventh transmembrane catalytic domain at the NH2 terminus, and are associated with an ectofacing peroxidase-like domain, which probably acts as a peroxidase. This isoform does not reguire a p22phox domain nor cytosolic components for its activation. It is becoming increasingly clear that an EF-hand binding protein (EFB1) interacts with the DUOX1/2 that allows the enzyme assembly. The DUOX enzymes are glycosylated isoforms. The activity of this enzyme is based on its glycosylation level. In partially glycosylated form, the enzyme generates superoxide anions, in mature glycosylated form, DUOX makes hydrogen peroxide, which is probably based on its peroxidase-like domain.
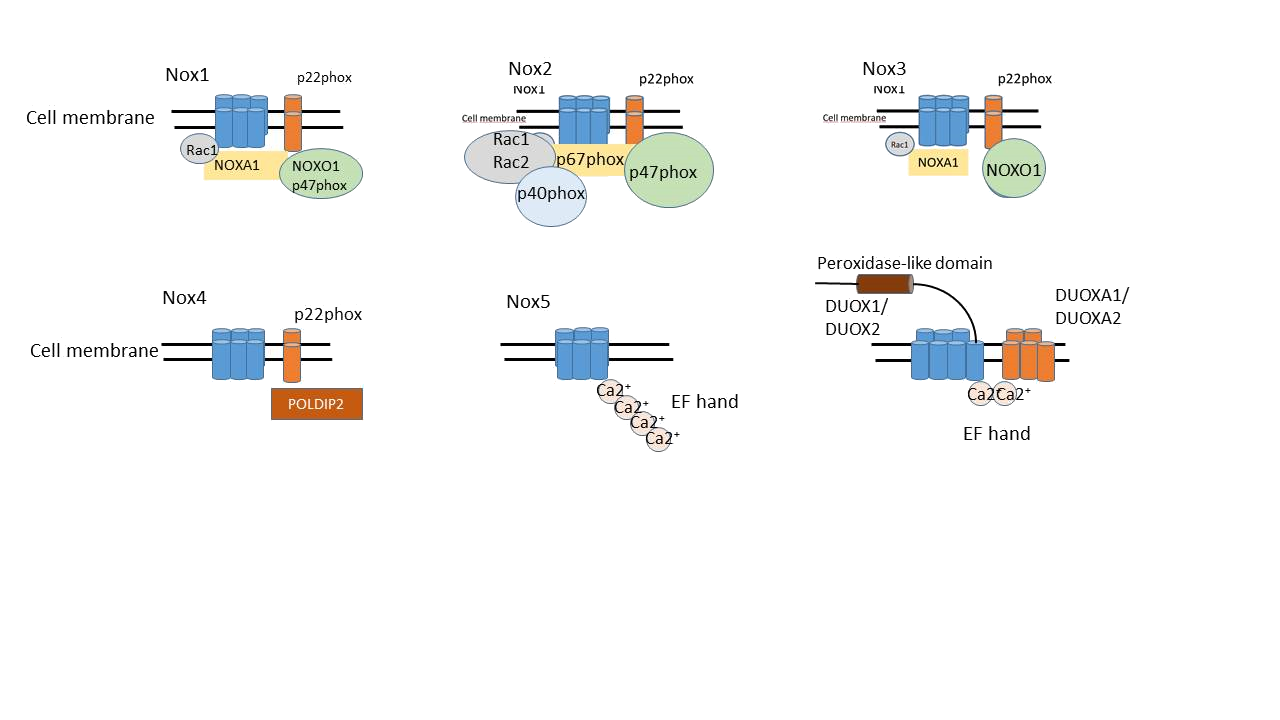
Figure 7: The discovered NADPH oxidase enzyme isoforms, Nox1, Nox2, Nox3, Nox4, Nox5, and DUOX1, DUOX2.
The tissue distribution of Nox homologs are well documented. The Nox1 is highly expressed in the gastrointestinal tract, especially in the colon epithelial cells, and other cell types, including endothelial cells, vascular smooth muscle cells. The prototypic Nox2 enzyme is tipycally expressed in phagocytic cells, like neutrophils and macrophages. There is increasing evidence that non-phagocytic cells also express Nox2, such as neurons, hepatocytes and cardiomyocites. Nox3 is the specific isoform in the inner ear, with especially high expression in the cochlear and vestibular epithelia and the spiral ganglion. Low levels of Nox3 also detected in fetal organs, fetal kidney and spleen. The discovery of Nox4 occurred in the kidney. Besides, Nox4 is also highly expressed in osteoclasts, smooth muscle cells, fibroblasts and neurons. High Nox5 mRNA expression is described in the testis, in vascular smooth muscle cells (VSMCs), in ovary and fetal tissues. DUOX1 and DUOX2 were found in tyroid salivary gland. They are also expressed in the colon, in airway epithelia and prostate.