The NO synthase dimer
All three NOSs share between 50 and 60% sequence homology. The NOSs exhibit a bidomain structure comprised of an N-terminal oxygenase domain that is linked to a C-terminal reductase domain through a calmodulin (CaM) binding motif. The reductase domains bind NADPH, FAD, and FMN, and the oxygenase domain contains binding sites for heme, tetrahydrobiopterin (H4B), and the substrates L-arginine and molecular oxygen. Dimerization of NOS proteins is essential for their activity. The interaction of two oxygenase domains creates an extensive interface between them. The 6R-tetrahydrobiopterin (H4B) cofactor interacts with residues in both subunits of the dimer and also hydrogen bonds to the active site heme. Dimerization activates NOS in at least three ways: it sequesters heme from solvent, creates high affinity binding sites for substrate l-Arg and cofactor, H4B, and enables electrons to transfer from the reductase domain flavins to the oxygenase domain heme. An essential feature of NOS is that, despite the ability of the reductase and oxygenase domains to function independently under certain circumstances.
NOS dimer formation may be an important point of biological and pharmacological regulation. A rank order for dimer strength of eNOS > nNOS > iNOS is suggested.
Role of Haem
The haem plays an essential role in dimerization (Fig. 12). In its absence, NOS exists as monomers which are essentially normal with respect to secondary structure. Furthermore, the ability to catalyse the NADPH-dependent reduction of cytochrome c is retained in nNOS monomers indicating that the transfer of electrons within the reductase domain from NADPH via the two flavins is not dependent on the dimeric structure. Monomers of all the isoforms are, however, unable to bind BH4 or a substrate analogue and do not catalyse L-citrulline/NO production. Haem is the sole cofactor for which there is an absolute requirement for the formation of active nNOS dimers, and it is also the key factor in eNOS dimerization. Although the characteristics differ from those of the two constitutive isoforms, the haem plays a similarly essential role in iNOS dimerization. The haem requirement for dimerization is common to all three NOS isoforms. They do, however, differ with respect to the role of BH4 in dimerization. Whereas nNOS and eNOS can form dimers in the absence of BH4, iNOS dimerization was reported to require the presence of the pteridine. Furthermore, BH4 stabilizes the nNOS and eNOS dimers once formed, and also the iNOS dimer, although not to the same extent. These data are supported by the reduced binding of BH4 by an N-terminal deletion mutant of iNOS, demonstrating the importance of residues 66–114 in iNOS for binding of the cofactor and hence dimerization. The close proximity of BH4 to the haem, as well as to the flavins at the domain–domain interface, hints at a possible role in electron transfer, although exogenously added BH4 does not appear to provide electrons for the reaction. In this respect, the role of BH4 in NOS differs from that in aromatic amino acid hydroxylation. A thorough analysis of the interaction of numerous pterins with iNOS revealed that the steps up to and including haem reduction are supported by dihydropterins as well as tetrahydro-pterins. However, only the latter are able to support NO synthesis and NADPH oxidation.
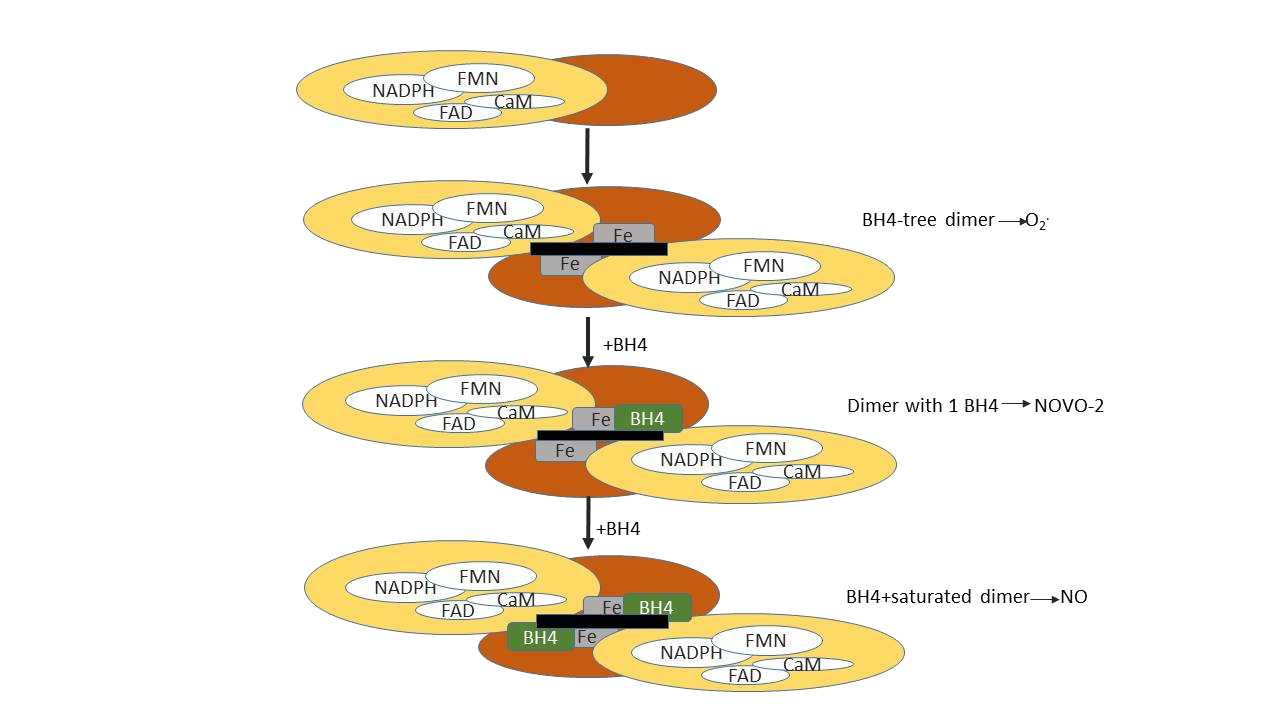
Figure 12: Stages of NOS dimer assembly.
Role of BH4
Tetrahydrobiopterin is a normal cofactor for a family of enzymes called monooxygenases. The tissue distribution of BH4 is consistent with the sites of monooxygenase activity, but there is little information available on the levels of this factor in the cells (endothelial and smooth muscle) of the blood vessel wall. A number of studies suggest that bound BH4 stabilizes the dimeric peptide structure of NO synthases, and this may be its mode of action. The most compelling evidence for this role comes from studies on the depression of cytokine-induced NO synthase activity by transforming growth factor-β (TGFβ).
Calcium dependence and the role of calmodulin
Calmodulin (CaM), a ubiquitous 17-kDa cytosolic protein, is a major cellular Ca2+ sensor which rapidly regulates intracellular processes through the co-ordinated activation of over 50 intracellular proteins. A CaM-binding domain separates the oxygenase and reductase regions in NOS enzymes. At raised Ca2+ concentrations, CaM binds to constitutive NOS (cNOS) enzymes, neuronal NOS (nNOS) and endothelial NOS (eNOS), enabling conformational changes in the reductase domains that facilitate electron transfer from NADPH through reductase-associated flavins to the catalytic heme in the oxygenase domain. Dependence on Ca2+ is a key distinguishing feature between the constitutive and inducible isoforms. eNOS and nNOS are both activated by an elevation in intracellular Ca2+, followed by the subsequent binding of Ca2+ CaM. In contrast, iNOS contains irreversibly bound CaM, and is hence largely independent of Ca2+, although a 2-fold greater activity is observed in the presence of 2.5 mM Ca2+ compared to that in 10 mM EGTA. The iNOS CaM-binding peptide enabled eNOS to bind CaM, but did not confer Ca2+ independence. Hence, only the Ca2+ bound conformation of CaM can activate the enzyme. The CaM-binding region in eNOS is directly involved in membrane association, specifically to anionic phospholipids such as phosphoserine, and this association prevents the binding of CaM to eNOS and hence catalytic activity. CaM binds to both the isolated reductase domain of nNOS as well as to the full-length enzyme, and stimulates the rate of electron transfer within the reductase domain. CaM is furthermore essential for the trans-domain transfer of electrons to the haem.