NADPH oxidases
Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH-oxidase, Nox) is a multi-subunit enzyme complex, which is expressed by phagocytic and non-phagocytic cells, such as cardiomyocites. Its main role, in mammals and also other organisms, to create reactive oxygen species (ROS) in a reaction which uses NADPH as electron donor, and molecular oxygen as electron acceptor. ROS, such as superoxide, hydroxyl, peroxyl and hydrogen peroxide, contain oxygen radicals, which are highly reactive. ROS level is increased when the oxidant-antioxidant system is imbalanced: during inflammation or endoplasmic reticulum stress (ER stress). The figure 5. shows the genesis of the reactive oxygen species, and the syntethizing enzymes. The reaction catalyzed by NADPH oxidase occurs by superoxide (O2-), which than forms hydrogen peroxide (H2O2), by the superoxide dismutase (SOD), and peroxidase enzymes. In Fenton reaction this H2O2 becomes a hydroxyl group (OH-), and eventually the catalase enzyme is required for protection of the cell from oxidative stress, transforming the radicals to water.
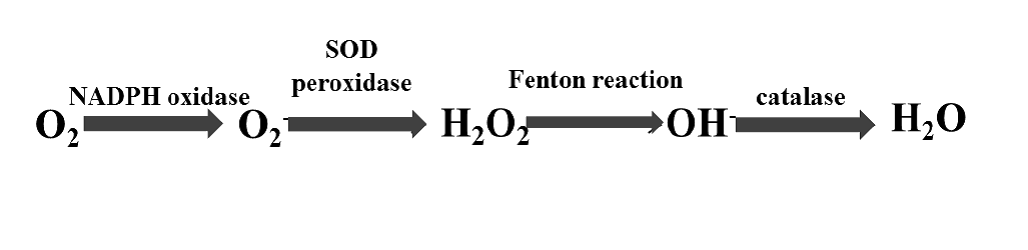
Figure. 5: The genesis of reactive oxygen species (ROS).
Over the past decades, the major ROS-generating NADPH oxidase enzyme’s several isoforms have been described, foremost the phagocityc Nox2 form (gp91phox) was identified. For an appropriate operation of the enzyme, not only a core catalytic domain is necessary, but also cytosolic „phox” (termed phagocytic oxidase) proteins (specifically p22phox, p40phox, p47phox and p67phox) and an Rho GTPase family member, Rac1 or Rac2 (Fig. 5 and 6).
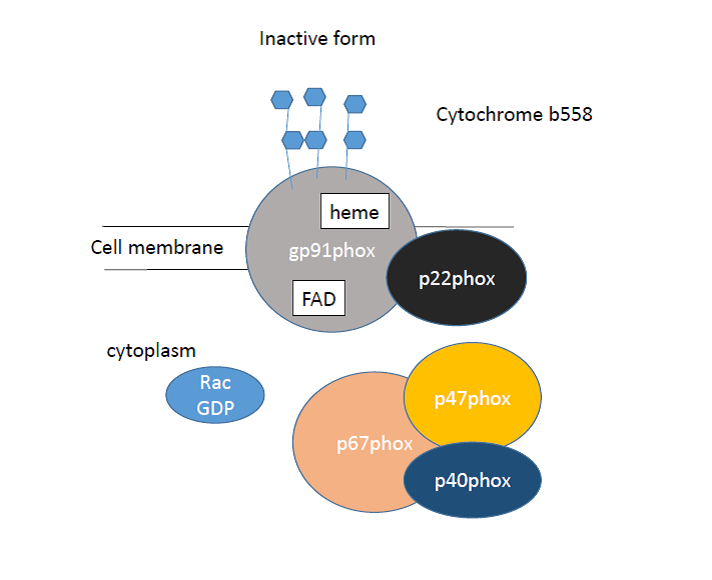
Figure. 6A: The inactive and the active, assembled form of NAPDH oxidases.
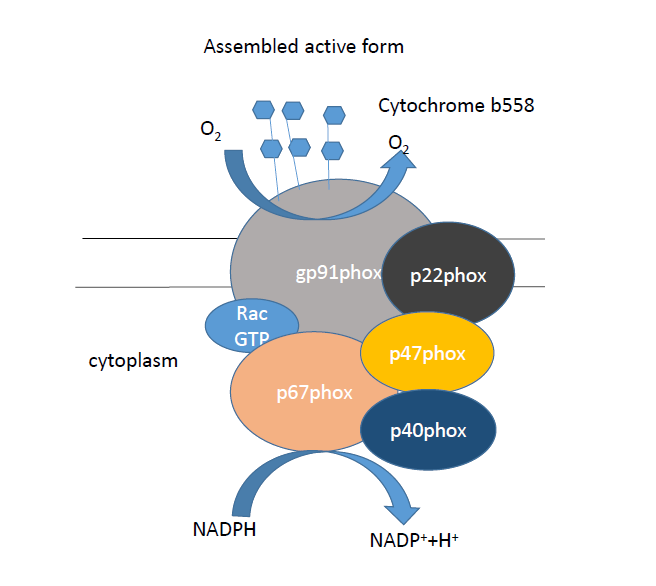
Figure. 6B: The inactive and the active, assembled form of NAPDH oxidases.
The core domain, placed transmembrane, in the plasma membrane or intracellular localization, is gp91phox and p22phox, which together forms cytochrome b558. The gp91phox is glycosylated, and contains two heme and a FAD molecule. The gp91phox N terminus contains six transmembrane alfa-helices, with two histidine residues. The C terminus comprise the FAD and the NADPH-binding sites. The p22phox is a non-glycosylated subunit, and its role is to help stabilize the gp91phox domain in the membrane, and anchors the p47phox when the enzyme is assembling. In resting cells, the catalytic domain is inactive and the cytosolic subunits form a three-protein complex in the cytoplasm. The gp91phox catalytic subunit used for its activation, need the translocation and bonding the cytosolic subunits.
The cytosolic complex contains p47phox, p67phox and p40phox. The p47phox is the „organizer domain”, the p67phox is termed as the „activator domain”, because of the mechanism below. In the activation of the enzyme, the 47kDa molecular weigth p47phox protein is phosphorylated by the protein kinase C (PKC). Because of this, the p47phox suffers conformational change, and thus p22phox and p47phox binding is enabled. This bond forms between p22phox C terminus prolin rich repeat (PRR) tail and p47phox two SRC homology 3 (SH3) domains. Accordingly, this event contributes to p47phox organizer function and also this domain is responsible for the translocation of the cytosolic activator p67phox domain to the catalytic domain. This subunit is not glycosylated, and contains a tetratricopeptide-repeat (TPR) at the N terminus, an activation domain (AD), a „Phox and Bem1 domain” (PB1), and a SH3 domain at C terminus. The p67phox also interacts with p47phox through the SH3 and PRR domains. The third protein of the cytosolic complex is p40phox, whose role is controversial, there are studies to prove both positive and negative effects. (p40phox: The last NADPH oxidase subunit). The p40phox is a non-glycosylated protein which has an SH3 domain, a PX domain, and a PB1 domain. This protein interacts with the p47phox and p67phox proteins. The final subunit which is important for the activation of NADPH oxidases, is the Rho GTPase family members Rac1 or Rac2. Rac is essential for the optimal ROS generation. In quiescent cells, the Rac is associated with a Rho GDP-dissociation inhibitor (GDI) protein, whose role is to keep the Rac protein soluble and inactive (GDP-bounded form) in the cytosol. In activated circumstances the GDP is replaced with a GTP by guanine nucleotide exchange factors (GEFs), thus the Rac is active, optimizing ROS generation.