Surface tension
|
|
Consider a drop of dew. Its shape is almost spherical. Why is it so? The explanation lies in a property of liquids called surface tension, which is a tendency to minimise the surface area. This phenomenon is only observable in liquids and is not present in gases. |
|
|
In liquids, there are attractive forces between molecules. A molecule in the bulk of the liquid is surrounded by neighbours from all sides, so the net force is zero. A molecule on the surface, however, has no neighbours above, and thus the net force acting on it will be directed towards the bulk of the liquid – that is, surface molecules experience a force that pulls them into the liquid. |
|
|
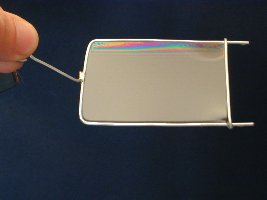
To quantify this effect, let us consider the setup in the picture: a wireframe with one mobile edge of length \(\ell\) contains a soap film. Due to surface tension, the molecules at the surface will be pulled into the liquid, so to keep the mobile edge in place, we have to exert a force \(F.\) This force is proportional to the length of the mobile edge, but it also depends on the type of the liquid. The constant of proportionality \(\gamma\) is called the surface tension, and it is a material constant. \[F = 2 \gamma \ell,\] where the factor 2 is there because the soap film has two surfaces. The SI unit of surface tension is the \(\frac{\mathrm{N}}{\mathrm{m}}.\) For instance, the surface tension of water (when surrounded by air) at 25 °C is \(72 \cdot 10^{-3}\,\frac{\mathrm{N}}{\mathrm{m}}.\) |